Abstract
Natural aqueous plant extracts are increasingly being used in biopesticide and cosmetic formulations due to their effectiveness and safety. However, these extracts are susceptible to fast degradation, which can lead to changes in color, odor, and efficacy. This article is about how to prevent such issues.
Introduction
The discovery of the strong potential of natural plant extracts in biopesticides and cosmetics has led to an increased interest in their use in recent years. At the same time, the demand for natural and organic products is being driven by a growing number of people who are concerned about the potential harm caused by synthetic chemicals.
To be a viable alternative to synthetic insecticides and cosmetic active ingredients, natural bioactive extracts must be affordable (based on plant materials readily available and cheap) and be simple to prepare (not requiring complex equipment and solvents which are either toxic or difficult to buy). Aqueous and ethanolic plant extracts are preferred for bioactive extracts. Maceration, soaking dried plant material in water or ethanol, yields a "macerate" for various uses. Other methods include solvent extraction, hydro-distillation, steam distillation (for fresh plants), and mechanical extraction.
However, natural aqueous plant extracts are prone to degradation, resulting in changes in color, odor, and efficacy. This susceptibility poses challenges in their use in cosmetic and biopesticide formulations.
The main culprits of degradation of the natural plant extracts are MAILLARD REACTION AND BIOCONTAMINATION, both arising from sugars present in aqueous media.
Color and Odor Development due to Maillard reaction
The Maillard reaction is a chemical reaction that occurs between amino acids and reducing sugars. It is responsible for the browning as well as the development of undesirable flavors and aromas in natural cosmetics and biopesticides. Some common examples of the Maillard reaction from the food, cosmetics and biopesticide production include: the browning of bread crust; the development of a bitter taste, flavor and specific fragrancy in beer and wine; the formation of off odors in cosmetics; the loss of efficacy of biopesticides.
The Maillard reaction occurs between amino acids and reducing sugars in the plant extracts to create melanoidins, the compounds which give browned extract its distinctive flavor. The reactive carbonyl groups of the sugars react with the nucleophilic amino groups of the amino acids and form a complex mixture of poorly characterized molecules responsible for a range of aromas and flavors. This reaction is the basis for many of the flavoring industry's recipes. It is well known that this phenomenon is responsible for the browning of food during cooking, as well as the development of flavor and aroma in many foods and beverages, such as browning of malted barley as found in malt whiskey and beer. The reaction is a form of non-enzymatic browning. It is named after French chemist Louis Camille Maillard, who first described it while attempting to reproduce biological protein synthesis.
The Maillard reaction can occur at any time, but it is most likely to occur when the product is exposed to heat, moisture, or oxygen. It can also occur during storage if the product is not properly packaged. The rate, extent, and course of Maillard reactions are influenced by several factors including, but not limited to, the type of amino acids and reducing sugars present, the temperature (which accelerate around 140 to 165 °C), the pH, time on the shelf and the presence of other molecules.
Preserving the Freshness – blocking Maillard reaction
The reaction can be prevented or slowed down by several methods, including the use of antioxidants, chelating agents, and heat treatment. Over the years, researchers have explored how to control Maillard reactions. The rate and extent of these reactions are influenced by environmental factors, which can affect multiple quality parameters, such as organoleptic properties, color, and protein functionality. Unique aroma profiles are developed dependent on temperature−time profiles used during processing. In some cases, Maillard reactions contribute to desired changes such as generation of delicate flavors, whereas in other cases undesired quality changes are obtained, especially if the Maillard reactions are too pronounced, producing bitter and burnt flavors. Thus, being able to control Maillard reactions during production and storage is important from a final product quality perspective. Some of the methods for controlling the Maillard reaction are:
- Using antioxidants, such as ascorbic acid or tocopherol
- Using chelating agents, such as Citric Acid or EDTA
- Protection of natural extracts in the formulation (microencapsulation)
- Packaging the product in an airtight container.
One of the common methods to control the Maillard reaction is to use antioxidants. Antioxidants can help to prevent the oxidation of amino acids and reducing sugars, which is a key step in the Maillard reaction. Common antioxidants may include Vitamin E (Tocopherol) - not only acts as an antioxidant to protect cosmetic products from oxidative deterioration but also offers potential skin benefits; Ascorbic Acid (Vitamin C) - has antioxidant properties and can enhance the stability of other ingredients.
Another method for addressing coloration is to use chelating agents. Chelation agents can bind to metal ions, which can catalyze the Maillard reaction. Common chelating agents may include: EDTA (Ethylenediaminetetraacetic Acid) - used to improve the stability of cosmetic formulations by binding to metal ions that can catalyze product degradation.
Use of microencapsulation techniques can help to preserve the extract from negative influence of the environment when the plant extracts are introduced into the final formulation. Microencapsulation involves encapsulating the extract in tiny particles, which are then dispersed in a carrier material.
Further packaging the final product in airtight containers is intended to limit the oxidation by air Oxygen.
KNOW-HOW
One of the popular methods to control Maillard reaction is used in beer and winemaking. This method can be successfully applied also in other aqueous plant extracts for cosmetic and biocide formulations. In beer and wine production, the Maillard reaction plays a very important role. It contributes to the development of flavor and aroma. It also helps to stabilize the beer and wine by forming brown pigments that protect the beer and wine from further oxidation. For control of the rate, extent, and course of Maillard reactions in beer and wine production, Sodium Metabisulfite (SMB) is often added. SMB (Na2S2O5) is a reducing agent that can react with amino acids and reducing sugars to stop the formation of brown pigments. In water solutions, Sodium Metabisulfite forms NaHSO3:
Na2S2O5 + H2O = 2 NaHSO3
The last releases sulfur dioxide SO2. Sulfur dioxide and sulfur (IV) oxo-anions in water solution undergo pH-dependent equilibration reactions between sulfur dioxide, sulfurous acid, bisulfite ion, and sulfite ion. Sulfur dioxide forms adducts by reversible action with aldehydes and ketones including reducing sugars, acetaldehyde, quinones, and ketoacids, that do not participate in the Maillard reaction. Anti-browning action of Sodium Metabisulfite is based on the blocking of Maillard reaction.
Color and Odor Development due to Biocontamination
Another reason for the instability of the formulations based on natural aqueous plant extracts is fast microbial contamination due to the presence of large amount of sugars in such extracts. This problem cannot be underestimated because it is a source of huge negative potential to deterioration of such formulations.
A key factor to assess the cleanliness and microbial contamination of products, equipment, and environments which is commonly used in various industries, is BIOBURDEN. Bioburden refers to the population of viable microorganisms, such as bacteria, fungi, and other microorganisms, present on a surface, in a substance, or in a specified environment. Manufacturers need to determine and control the level of bioburden to prevent microbial growth that could compromise the safety and efficacy of these products.
Bioburden testing typically involves collecting samples, culturing microorganisms under specific conditions to encourage their growth, and then counting and identifying the resulting colonies. The results of bioburden testing are used to set acceptable limits for microbial contamination and to establish appropriate sterilization and disinfection processes.
In cosmetic, food and medical industries a Total Bacterial Count is used for monitoring the bioburden. It indicates how many microorganisms are present in a sample. Monitoring the total bacteria count is necessary, because the number of microorganisms shouldn't exceed certain guide values. These guide values, which are expressed in CFU (colony-forming units) per gram or milliliter, depend on the different types of the products.
The two most widely used methods for determining bacterial numbers are the standard, or viable, plate count method and spectrophotometric (turbidimetric) analysis.
KNOW-HOW
However, for more “rough” production, like pesticide industry, a simple method for bacterial count estimation can be applied. The method utilizes ready-to-use medium sheets that are coated with a patented blend of dry culture media and non-woven fabric. This makes it ideal for a variety of microbiological routine testing applications.
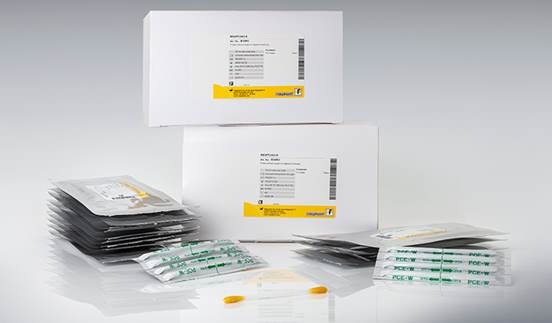
After application the medium sheets are incubated at specified temperatures for 24-48 h. The colonies grown on the surface of the medium sheets can be counted easily after incubation. For analysis one should open the transparent cover film and apply 1 ml of the aqueous extract / formulation onto a medium sheet and close the film again. Then allow 10-30 minutes for complete absorption before use. Incubate the medium sheets at specified conditions at 35°C for 24-48 hrs. and then count the colonies grown on the surface of the medium sheet after incubation comparing appearance of dots on the sheet with those given in the instruction. Such simple testing does not necessitate the use of a clean room or sterile hood.
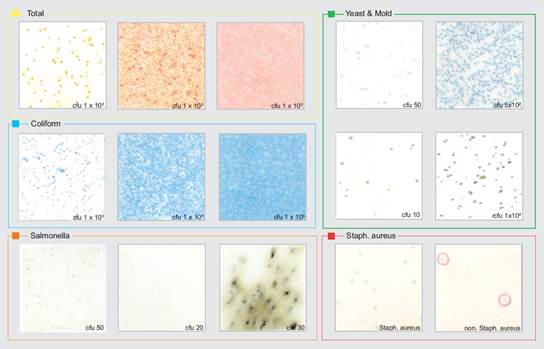
Preserving the Freshness – controlling biocontamination
As mentioned, apart from the Maillard reaction, natural plant extracts are highly prone to being contaminated by microbes. This susceptibility can result in the proliferation of bacteria, fungi, and other tiny organisms, causing the product to spoil and rendering it unfit for use. Various biocides are regularly employed in the cosmetics and biopesticide industries to hinder such microbial contamination. Another effective method for preventing additional microbial contamination involves utilizing alcohols or their combinations with water during the preparation of plant extracts. To prevent microbial contamination the following methods are applied:
- Using biocides against microbial contamination
- Sterilization
When considering biocides for use in the cosmetic industry, here are some commonly used for the aqueous plant extracts in cosmetic formulation:
- Phenoxyethanol: A widely used preservative with broad-spectrum antimicrobial activity.
- Sodium Benzoate: Effective against fungi, bacteria, and yeast. Often used in combination with other preservatives.
- Potassium Sorbate: Inhibits the growth of molds and yeasts. Often used in conjunction with other preservatives.
- Quaternary Ammonium Compounds: Benzalkonium Chloride (BACs): Commonly used in personal care products for its antimicrobial properties.
- Alcohol: Ethanol or Isopropyl Alcohol: Can be used as a co-solvent with water and disinfectant or antimicrobial agent in aqueous plant extracts
- Natural Biocides:
- Tea Tree Oil: Possesses natural antimicrobial properties and is often used in organic or natural cosmetics.
- Grapefruit Seed Extract: Claimed to have antimicrobial effects and is sometimes used as a natural preservative.
When it comes to pesticide formulations, the selection of biocides is critical for ensuring the effectiveness, stability, and safety of the product. Here are some commonly used biocides in pesticide formulations:
- Benzalkonium Chloride: A quaternary ammonium compound with broad-spectrum antimicrobial activity. It is effective against bacteria, fungi, and algae and is commonly used in liquid pesticide formulations.
- Bronopol: 2-Bromo-2-nitro-1,3-propanediol, is an organic compound with wide-spectrum antimicrobial properties.
- 1,2-Benzisothiazol-3-(2H)-One (BIT): BIT is a broad-spectrum antimicrobial biocide which exhibits rapid inhibition of growth at very low levels and biocidal effects at higher levels.
- 2,2-Dibromo-3-Nitrilopropionamide (DBNPA): This biocide is effective against a wide range of microorganisms, including bacteria, fungi, and algae. It's often used in water-based pesticide formulations.
Additional information on preventing biocontamination in pesticide formulations you may found in the article “Mitigating microbial contamination in aqueous SC formulations. Risks and preventing strategies”:
https://michberk.com/mitigatingmicrobialcontamination.aspx
In addition to using biocides, the natural extracts in a formulation can also be protected by sterilization, which is the process of killing all microorganisms in a product. To achieve sterility, a holding time of at least 15 min at 121°C at 100 kPa (15 psi) or 3 min at 134 °C at 100 kPa (15 psi) is required. Additional sterilizing time can be required for packed liquids as they may take longer to reach the required temperature. However, such conditions can be destructive for natural plant extracts, and can promote the Maillard reaction.
KNOW-HOW
Sterilization at normal temperature can be performed by filtration through a membrane with 0.2- or 0.1-micron pore size. Such filtration removes biological contaminants, including bacteria, mold, and yeast. This method applied to the plant extracts more likely should also require pre-filtration through higher pores filters (1, 2 or 5 microns) in sequence. It requires use of sterile equipment and tools as well.
KNOW-HOW
A milder process that kills most microorganisms, but not all, is PASTEURIZATION. Pasteurization is the treatment of a product to make it safe for consumption and to improve its shelf life. Unlike sterilization, which uses high-temperature treatment to eliminate all microorganisms, resulting in a product that can be stored indefinitely at room temperature, pasteurization is carried out at lower temperatures and aims to reduce the overall microbial population to acceptable levels that can be maintained at refrigerated temperatures.
The main purpose of pasteurization is to reduce the “bioburden” of the product. The principle of the pasteurization procedure resides in reducing the bioburden by applying a sequence of heating steps for a defined amount of time, for example very often pasteurization require 5-log cycles at 63°C for at least 30 min., or at 72°C for at least 15 sec. A key parameter in designing a pasteurization process is determining the optimal duration of heat application that is needed to achieve the desired level of microbial killing (desired bioburden level).
Conclusion
Natural plant extracts are a valuable resource for bio-pesticides and cosmetic formulations. However, exploiting the potential of these extracts depends on effectively addressing the problems associated with MAILLARD REACTION AND BIOCONTAMINATION. Strategic approaches such as the use of antioxidants or Sodium Metabisulfite to slow down Maillard reaction, as well as the use of biocides and pasteurization techniques to regulate bioburden, have proven highly effective in maintaining the integrity of these extracts. Combined with the convenience of application of ready-to-use medium sheets for simple bacterial count, these strategies may play a key role in maintaining the freshness and efficiency of extracts.
Furthermore, the robustness of natural plant extracts can be influenced by various supplementary factors. These factors encompass the type of plant material, the technique used for extraction, how they are stored, and the presence of extra components. Diligent attention to these aspects holds immense significance when crafting biopesticides and cosmetics derived from natural plant extracts. By conscientiously implementing strategies to uphold the vitality of these extracts, you can assuredly ensure their efficacy and safety.