Controlled-release fertilizers (CRFs) are another significant facet of agricultural practices. These granular fertilizers are coated with polymers, such as polyurethane, polyolefin, and polyvinylidene chloride, to facilitate gradual nutrient release over time through osmotic pressure.
Remarkably, the intentional addition of microplastics to fertilizers, CS pesticides, and Seed Coatings has become a pressing concern. A 2019 report by the European Chemicals Agency (ECHA) revealed that nearly half of the estimated 51,500 tons of microplastics used annually in the European Economic Area (EEA) were intentionally added to agricultural products. Of this total, approximately 22,500 tons were found in fertilizers, 500 tons in pesticides, and another 500 tons in seed coatings.
Globally, estimates for the quantity of intentionally added microplastics in the fertilizer sector exhibit significant variation. According to a major report by the United Nations Food and Agriculture Organization (FAO) on agricultural plastics in 2021, polymer-coated fertilizers account for around 100,000 tons of plastic used in agriculture each year. However, self-reported data from prominent nitrogen fertilizer manufacturers challenge this figure. For example, Nutrien claims an annual production of over 400,000 tons of polymer-coated fertilizer, while ICL Specialty Fertilizers' annual production capacity for CRFs reaches approximately 200,000 short tons. Additionally, Kingenta reported a production capacity of approximately 1.7 million tons of CRFs in 2015. When considering the reported production totals from just these three companies, it becomes evident that the global production and use of intentionally added microplastics might be far greater than currently recognized.
Consequently, the presence of MICROPLASTICS poses a mutual challenge for CS pesticides and CRF fertilizers. Microplastic coatings and polymer shells undergo fragmentation, leading to the generation of very small-sized plastic debris. These particles can even reach the nanoscale level, falling within the range of nanoplastics (1 - 1000 nm). Such synthetic polymer materials exhibit poor biodegradation, leading to their persistence in the soil environment for extended periods. As a result, plastic particles accumulate at alarming rates, reaching up to 50 kilograms per hectare per year, thereby polluting the soil and becoming susceptible to dispersion through air, water, and other vectors. Finding effective solutions to mitigate the impact of microplastics on agricultural practices and the environment remains an urgent necessity.
-
Polymer Coating in CRF. Controlled Release Fertilizers (CRFs) consist of round granules composed of water-soluble salts, encased in a porous multilayer polymer coating. These coatings are typically made of polyurethane, polyolefin, and polyvinylidene chloride, facilitating the gradual release of fertilizer contents over time through osmotic pressure.
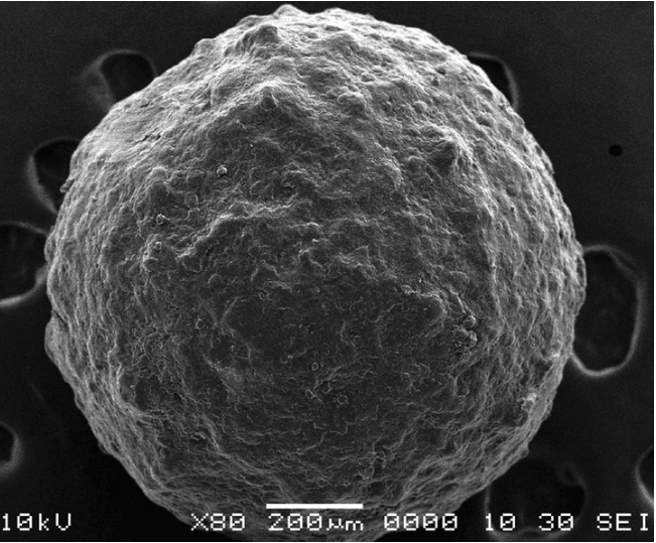
Fig. 1 Scanning electron microscopy (SEM) photo of CRF polymer coated pellet.
The release mechanism of these fertilizers relies on the process of diffusion. Water vapors permeate the polymer coating from the external environment, condense beneath the shell, and dissolve the top layer of solid salt within the granule. The concentration gradient of the salt between the saturated aqueous solution under the shell and the zero concentration outside the granule drives the diffusion of the fertilizer solution through the water-permeable coating, facilitated by osmotic pressure. As the solid fertilizer gradually dissolves, the space under the shell is filled with water until the entire internal area beneath the coating becomes water-saturated.
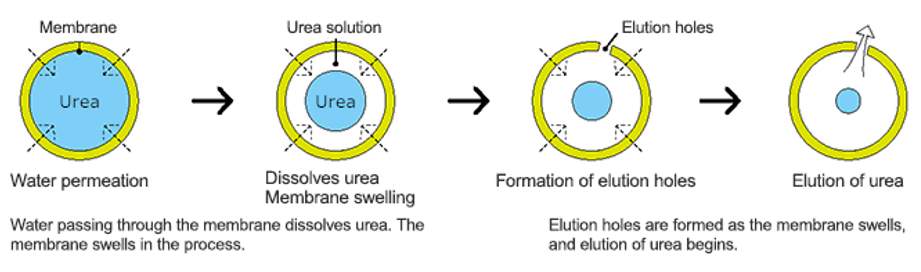
Fig.2 Mechanism of nutrients release from coated CRF granules.
The time of nutrient release from CRFs is regulated by the thickness and permeability of the polymer coating, as well as by environmental factors such as humidity and temperature.
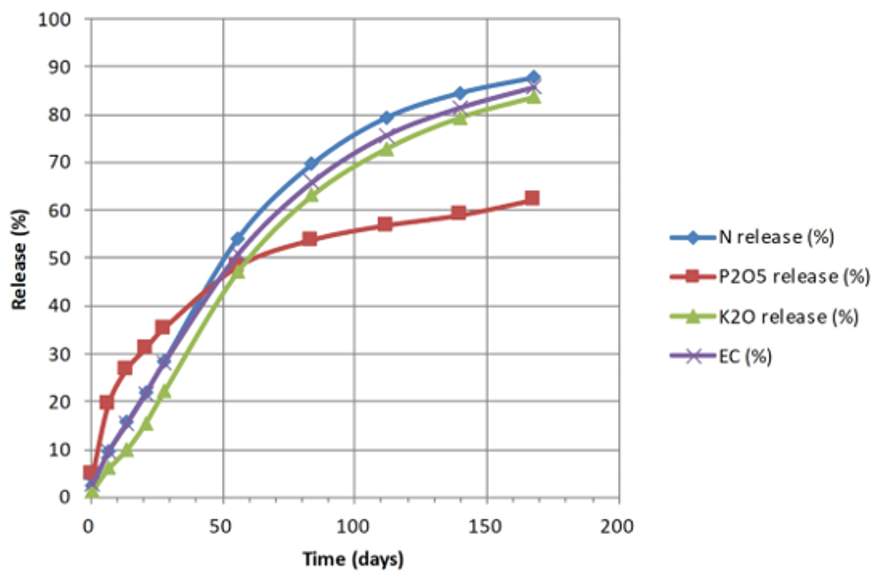
Chart 1. Example of controlled release of N, P, K from “Osmocote” product.
To communicate this complex process more simply to end-users, electrical conductivity (EC) is commonly used to define the release duration of these products. The duration required to reach higher EC values correlates with the average release rates of various nutrients, providing a convenient and indicative measure of their gradual release over time.
-
Polymer encapsulation in CS pesticide formulations. According to pesticide experts, synthetic polymer encapsulation, originally intended as a protective barrier to reduce skin contact with highly toxic pesticides, has evolved for use in delayed- or controlled-release functions. Some industry players promote controlled-release or "enhanced-efficiency" pesticides as a potential solution to mitigate the pollution and other negative impacts associated with synthetic chemicals in Crop Protection Products (CPPs), often marketing them under the umbrella terms of "precision" or "climate-smart" agriculture. However, the veracity of such claims remains questionable.
The process of microencapsulation involves enveloping an Active Ingredient (A.I.) within a polymer shell, often composed of polyureas and polyurethanes, and filled with the pesticide's active ingredient, usually dissolved in a water-insoluble solvent. Ensuring the stability of Capsule Suspension pesticides during storage relies on maintaining all active materials inside the capsules throughout the storage period. Qualified CS formulations should contain no more than 0.1% of A.I. outside the capsules. A higher concentration of A.I. in the aqueous medium outside the capsules can lead to various issues in the future:
- Crystal growth of the active substance in water, resulting in problems during field application, such as clogging the filters and meshes in the spray system.
- Sedimentation of A.I. in the package, making it difficult to empty the entire active substance, and subsequently reducing the A.I. concentration in the spray mixture.
- Release of the active substance from the capsules during storage, indicating capsule shell degradation. This leads to polymer shell residues contaminating the formulation, exacerbating pollution issues within the spray system.
The bio-efficacy of CS formulations in the field is a critical concern, particularly regarding the mechanism of A.I. release after field spraying. Several factors influence this release mechanism from the polymer capsules, including:
- Structure, porosity, and strength of the polymer shell.
- The aggregate form of A.I. inside the capsule.
- The solvent which is used to dissolve A.I. in the organic phase.
- Concentration of A.I. inside the capsule.
- Osmotic pressure.
- Environmental conditions outside the capsules, such as water, humid air, dry air, and exposure to UV.
The behavior of polymer shells in different environments varies.
In dry atmosphere capsules tend to break down, depending on factors such as shell thickness, strength, and resistance to UV. See Fig.3.
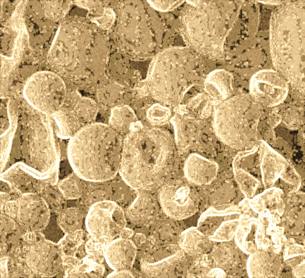
Fig. 3 Partially disrupted and deformed capsules
In a humid environment, well-preserved, robust, and intact non-defective capsules are commonly observed (See Fig. 4).
Fig.4 Well-preserved whole capsules
After field application, a mixture of broken, deformed, and well-preserved whole capsules is typically observed at various times. This variation depends on external factors, such as weather and irrigation, as well as the formulation's stability, storage time, storage conditions, and the integrity of the capsules before spraying.
Three known mechanisms govern the release of the Active Ingredient (A.I.) from the capsule.
In instances of significant dilution of the Capsule Suspension (CS) formulation with water, diffusion of A.I. through the polymer shell takes place. The rate of diffusion is influenced by the thickness and porosity of the shell, the affinity of the solvent used in the organic phase to the polymer shell, the concentration of A.I. in the organic phase, and the level of dilution of the formulation. In most cases, the release of A.I. is relatively rapid, especially when the diluted composition is sprayed in wet field conditions (See Chart 2).
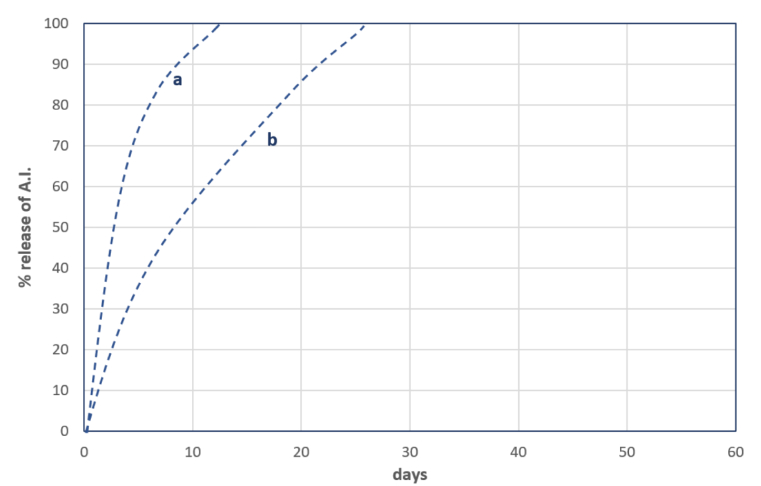
Chart 2.
a - less solvent in the capsule or better affinity of the solvent to the shell polymer,
b - more solvent in the capsule or the lesser affinity of the solvent to the shell polymer
When the CS formulation is sprayed in a dry environment, the capsules undergo rapid drying under the direct influence of the sun. Some of the capsules will rupture, resembling "popcorn," resulting in the release of A.I. from the broken capsules, making it biologically available. However, this phenomenon occurs only with a portion of the capsules and depends on their size, strength, and resistance to UV exposure. Consequently, only a fraction of the A.I. will be released in such conditions (See Chart 3).
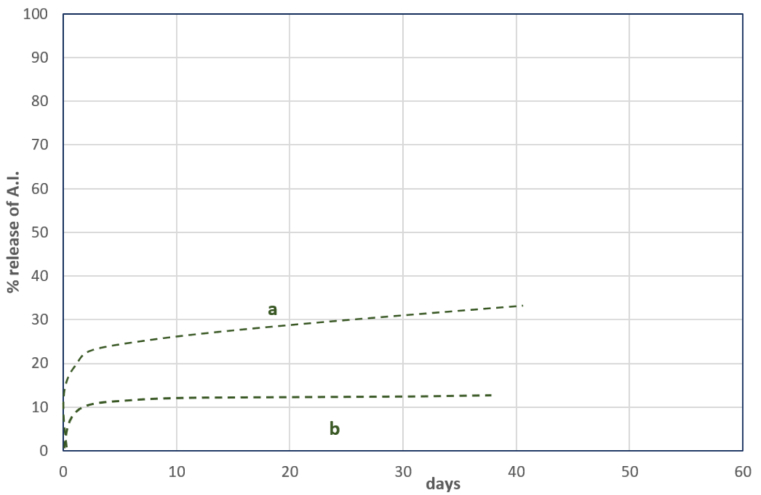
Chart 3
a - the thin wall of the capsule
b - the thick wall of the capsule
Typically, there is a mixed release of A.I., involving both the diffusion mechanism and capsule disruption, occurring in varying ratios depending on unpredictable weather conditions and the state of the capsules in the formulation
(See Chart 4).
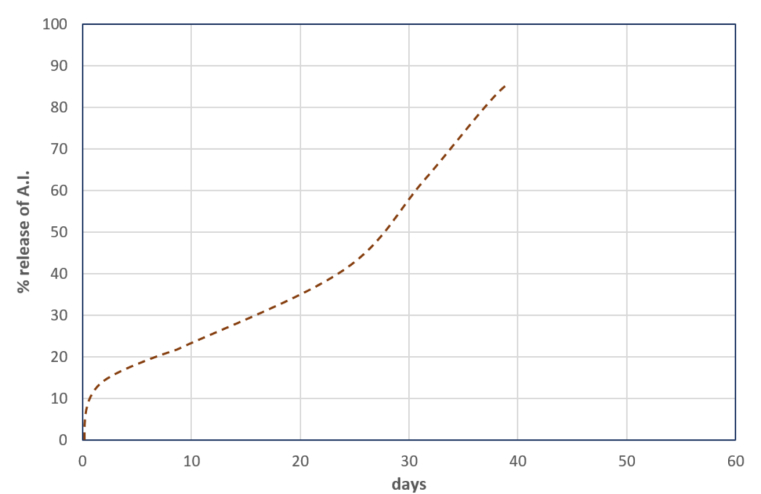
Chart 4
Thus, when the slow release of A.I. is touted as an important feature of CS formulations, it's essential to understand that such slow release is unpredictable and cannot be likened to the controlled release seen in CR fertilizers. This gap is even acknowledged by the industry, as stated in a 2020 publication by the IFA: "Due to the intrinsic nature of these products, it is not always possible to test the release under controlled laboratory conditions in such a way that a release property under field conditions can be automatically determined or predicted."
Furthermore, in the context of pesticides, the occurrence of a specific pest or disease in a particular field and the duration of protection required are unknown. Additionally, rain fastness further adds to the uncertainty surrounding the release potential of CS pesticides. Hence, unlike CR fertilizers, the potential need for "controlled release" of a pesticide formulation remains uncertain.
On the contrary, the uncontrolled release of A.I. from CS formulations can lead to efficiency problems when applied in the field. The effective dose of A.I. and the required amount of water per hectare for a specific crop and disease are always defined in the formulation label and approved by local regulations. This dose is calibrated for fully bioavailable A.I. However, when CS is applied, the A.I. may not be fully available due to uncontrolled slow release. As a consequence, this can significantly diminish its efficiency in the field.
-
Regulation aspect for CRF and CS pesticides. A trigger for advancing microplastics regulations in the coming years is the increasing requirement for their biodegradability. Currently, the absence of restrictions on plastic usage in agrochemicals means that plastic-coated fertilizers and pesticides will continue to contribute to the proliferation of microplastic pollution in soils and the wider environment, posing risks to both ecosystems and human health.
In response to this mounting concern, the European Commission's 2019 Fertilizing Products Regulation has taken a proactive step by imposing restrictions on polymer-coated fertilizers, mandating compliance with biodegradability criteria. This restriction will be enforced in all EU Member States by 2026. It is expected that other jurisdictions outside the EU will follow suit and take decisive steps to address the intentional use of microplastics in products, particularly in coated fertilizers and pesticides. So, too, international institutions, such as the World Health Organization, follow the lead of organizations like the FAO, which recommended banning non-biodegradable polymer-coated fertilizers, seeds, and pesticides in their 2021 report on agricultural plastics.
Critical regions for regulatory action include the United States and Canada, where the consumption of polymer-coated fertilizers has seen significant growth. Canada has initiated a new rule requiring the registration of fertilizer products containing polymers, indicating a heightened awareness of the issue. However, this regulation falls short of restricting or prohibiting the use of synthetic polymers in fertilizers. Likewise, Asia, particularly China, emerges as another major market for these products, with evidence pointing to the accumulation of microplastics, specifically from coated fertilizers, in agricultural soil, including paddy fields in Japan.
-
Future fate of CRFs and CS pesticides. The slow and uneven progress of international microplastics regulations, which emphasize the requirement for biodegradability in CRF and CS Pesticides, faces significant opposition from agrochemical manufacturers and agricultural practices. This is primarily due to the limited availability of such biodegradable products currently in the market. Although there is a strong request for biodegradable solutions in modern Agrochemical R&D, such innovative and effective products are not yet widely accessible.
Nevertheless, the growing influence of the green environmental trend is becoming increasingly apparent, setting the tone for modern agrochemical research. The mounting pressure to adopt sustainable practices is shaping the future landscape of the CRF segment in the fertilizers and micronutrients market. In the coming years, the future of the CRF segment in the fertilizers and micronutrients market is closely linked to biodegradable products with well-defined controlled-release properties that have been proven over decades of use.
When discussing CS pesticide formulations, the claim of necessity of controlled release for field application has not been substantiated in agricultural practice, often serving as more of a marketing gimmick. The primary purpose of such formulations remains vital—to protect farmers or industrial workers from exposure to particularly harmful A.I.s.
The emergence of the new need for CS pesticides in recent years is driven by the requirement to combine different A.I.s into a single formulation or various formulations in one tank mix, at same time avoiding potential issues of incompatibility. Encapsulation has become a practical solution to isolate incompatible A.I.s from one another, making it a valuable option.
The introduction of combined formulation types, like ZC (SC+CS), has become increasingly relevant to address these challenges. However, akin to CRF, the future fate of CS pesticides will be defined by meeting strong requirements for the biodegradability of the polymer capsule shell.
At the same time, the biodegradability of a successful CS composition should harmoniously coexist with its stability during a long term storage prior to subsequent field application, ensuring the preservation of the A.I.'s bio-efficacy. To achieve this, it must meet essential physicochemical criteria, enabling the rapid release of 100% A.I. (within 1-2 days) after spraying in the field—thus, a crucial factor in ensuring effective pest and disease control.
Such physicochemical criteria for a successful CS composition are as follows:
- The polymer shell must possess sufficient strength to maintain the capsules' integrity during storage, preventing A.I. diffusion from the capsules and preserving the polymer shell's structure.
- The quantity of A.I. outside the capsules should not exceed 0.1% of the total A.I. content in the composition.
- In cases where the composition is heavily diluted in the spray tank, the active substance must promptly diffuse from the capsules after spraying.
- After application and exposure to direct sunlight in the open air, the capsule's shell should rapidly degrade, facilitating the swift release of A.I. from the capsules.
In conclusion, the future fate of CRF and CS pesticides lies in the imperative development and adoption of biodegradable products. By embracing biodegradable alternatives, we will move towards a more environmentally responsible approach, safeguarding both agricultural productivity and the well-being of our ecosystems.