SOLVING QUALITY AND PRODUCTION PROBLEMS OF CROP PROTECTION FORMULATIONS.
A SYSTEMATIC APPROACH
Abstract
The article outlines a systematic approach to address both quality and production problems in crop protection formulations. This approach includes prioritizing the issues, defining the problems, specifying the formulation, creating an investigation plan, conducting research and discussions, and implementing improvement actions.
Introduction
Crop protection formulations play a vital role in ensuring the quality and quantity of agricultural production. However, quality and production problems always occur during the formulation process, resulting in rejected batches, customer complaints, and decreased profitability. This article presents a systematic approach to resolving these problems
-
Prioritization of the problems
The first step in the approach is to prioritize the problems based on the cost of the formulation, cost of complaints, amount of rejected batches, and possibility of reworking the rejected batches. This step helps in identifying the most critical problems that need immediate attention. To summarize the prioritization step, the collected data can be presented in a table format for easier analysis and comprehension.
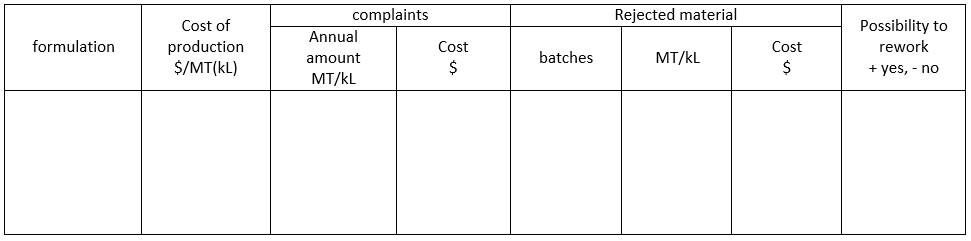
-
Problem definition
The second step involves defining the quality and production problems in detail. Quality problems can be defined based on the parameters that are out of specification after production or during the shelf life of the formulation. Manufacturing problems can be defined based on the production process steps and the parameters that deviate from the standard values.
-
Quality problem
To provide a clear understanding of the quality problem in the formulation under consideration, detailed definitions can be presented in an appropriate format, such as a table. This will help in organizing the information and make it easier to analyze. An example of the table format that can be used is provided below:
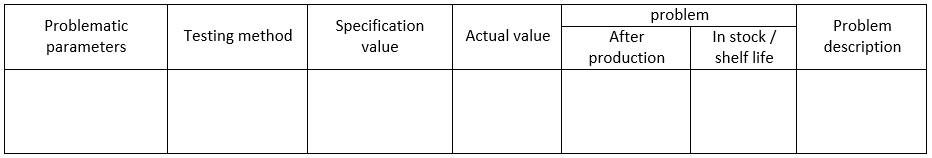
The above table provides a structure for presenting the problematic parameters, the testing method used to evaluate them, the specified value, the actual value, the problem, and a description of the problem. This format can be customized based on the specific details of the formulation under consideration and the quality problem being addressed.
-
Manufacturing problem
To better understand the production process problem, a detailed definition of the problem can be presented in a table format as shown below:
Formulation:
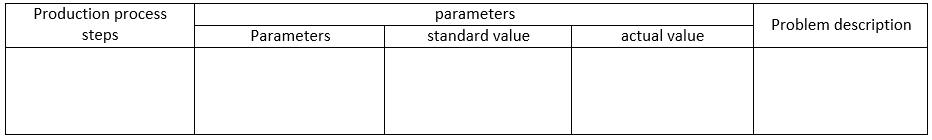
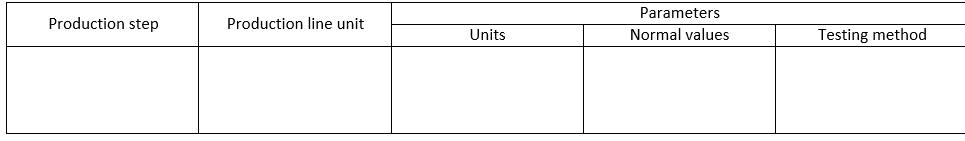
The above table provides a structure for presenting the production process steps, the parameters involved in the process, and a description of the problem encountered.
-
Formulation specification
The third step involves specifying the composition, quality parameters, and process parameters of the formulation. This step helps in identifying the potential causes of the quality and production problems. This can be also presented in a following table format:
-
Composition
Formulation:
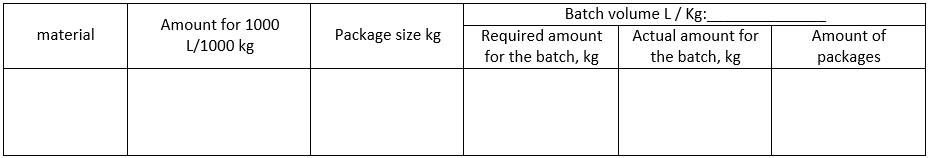
-
Quality parameters
Formulation:

-
Process parameters
Formulation:
-
Investigation plan
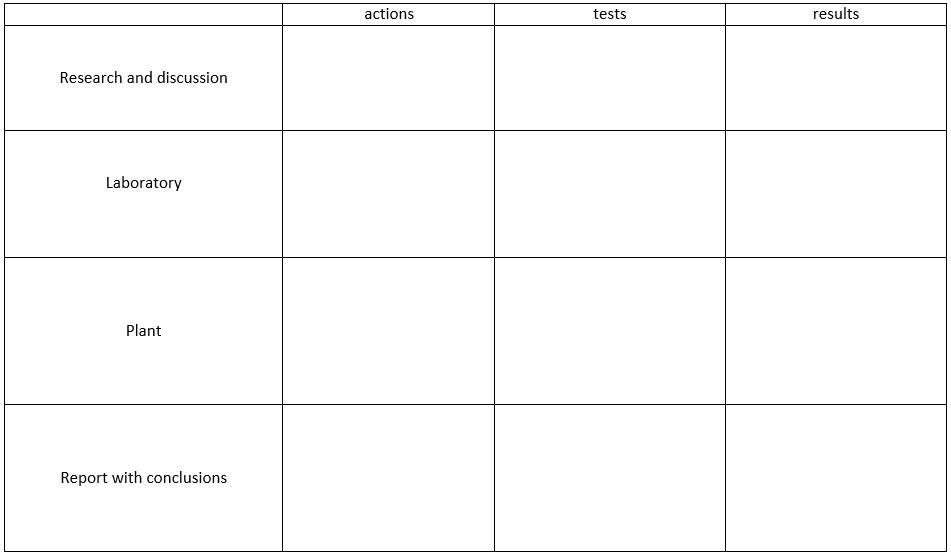
In the fourth step, an investigation plan is created to identify the root cause of the problems by outlining the necessary actions and tests. This step involves conducting the investigation, analyzing the results, and discussing the findings with the laboratory and plant personnel to develop solutions for the issues identified. Once the investigation is complete, the step concludes with the creation of a report containing conclusions for arranging further actions to implement the solutions and prevent the recurrence of the problems.
-
Further actions
After following the systematic approach outlined above and based on the results obtained during the investigation, it is necessary to arrange further actions to implement the identified solutions. These actions may include training, process improvement, and continuous monitoring of the formulation process, among others.
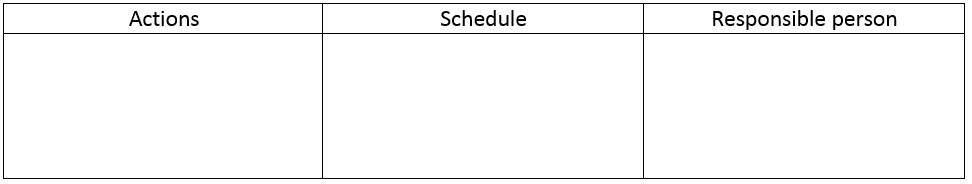
The implementation of these actions is expected to streamline the production process, improve the quality of the formulations, and reduce the number of complaints and rejected batches. To ensure the successful implementation of the identified solutions, the further actions should be outlined in a detailed plan that specifies the responsible person and schedule for their completion.
The plan should prioritize the most critical actions that need to be taken and provide a timeline for when they will be completed. By following this plan, the identified solutions should be successfully implemented, and the production process will be improved in a sustainable manner.
-
Conclusion
The comprehensive and systematic approach outlined above for solving quality and production problems of crop protection formulations places great emphasis on the importance of collaboration between laboratory and plant personnel. Through the implementation of this approach, the primary goal is to streamline the production process, enhance the quality of the formulations, and minimize complaints and rejected batches. The successful implementation of the solutions identified during the investigation is not only aimed at improving the current production process but also creating a sustainable solution for long-term success. The overall objective of this approach is to optimize the quality and efficacy of crop protection formulations in the most effective manner possible.