NB: OPEN KNOW-HOW
MANAGING CAKING ABILITY OF POWDER FERTILIZER BLENDS
Abstract
The caking ability of powder NPK fertilizer blends significantly impacts their handling, transport, and overall performance. This article highlights the best practice for evaluating and controlling the caking ability of NPK blends. The author offers comprehensive support to assist with implementation of this approach, contact the author.
CAKING
Agriculture relies heavily on a variety of fertilizers, with powder mineral blends being the most widely used. These blends typically consist of Ammonia, Urea, Nitrogen, and Phosphorus salts, including Potassium Nitrate, Ammonium Nitrate, Calcium Nitrate, Sodium Nitrate, Mono Ammonium Phosphate (MAP), Di Ammonium Phosphate (DAP), Mono Calcium Phosphate or Triple Super Phosphate (TSP), Single Super Phosphate (SSP), Urea, Ammonium Sulfate, Ammonium Chloride, Potassium Sulfate, Potassium Chloride, and K-MAG. The blends are identified by the letters NPK followed by numbers, which indicate the percentage of Nitrogen, Phosphorus, and Potassium.
While NPK blends are essential for plant growth, they can be challenging to handle and process due to their tendency to agglomerate and cake. This presents a significant challenge to both manufacturers and farmers who move and store millions of tons of these fertilizers each year. You can explore methods for controlling flowability of the solid fertilizer blends offered on this page: https://michberk.com/controllingflowabilityofsolidfertilizerblends.aspx
Caking is the result of fertilizer particles or granules sticking together due to contact points. It occurs during storage and is characterized by the formation of undesirable clumps. These contact points initiate reactions that are influenced by environmental factors, which lead to the dramatic formation of caking. The process is primarily driven by the formation of liquid and solid bridges caused by capillary condensation, chemical reactions, dissolution, and molecular attractions within the fertilizer structure, as depicted in the figure below.
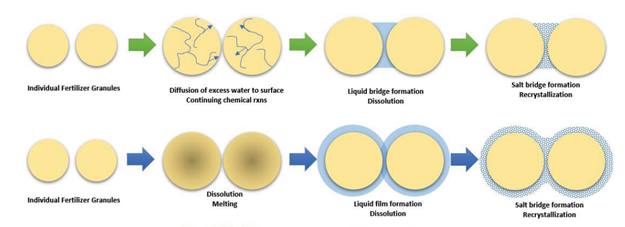
In order to prevent caking, it is necessary to assess and adjust certain fundamental properties of solid fertilizers such as moisture content, chemical composition, CRH, particle size, and other relevant factors.
The critical relative humidity (CRH) of a solid salt refers to the air relative humidity level at which the salt starts to absorb moisture from the surrounding air and undergoes a significant change in its physical properties, and may become damp or dissolve. The CRH value is specific to each type of salt and is influenced by factors such as temperature and chemical composition.
Apart from the free water content, the presence of dissolved ions in the water also contributes to the likelihood of caking. When ions are present in relatively high concentrations, they can create crystalline bridges on the surface of the granules, leading to caking. Ammonium and chloride ions, in particular, have been found to be associated with high caking tendencies in various studies. This is due to the fact that these ions react on the surface of the granules, resulting in the formation of ammonium chloride salt.
KCl + NH4NO3 -> KNO3 + NH4Cl
The caking in fertilizer can be influenced by its chemical composition. For example, fertilizers containing ammonium nitrate and urea have a greater tendency to cake than those containing ammonium sulfate or ammonium phosphate. One of the main causes of caking is the formation of ammonium chloride, which occurs when a saturated ammonium chloride solution evaporates on the outer surface of the fertilizer granule and creates salt bridges with nearby components. The source of these salt bridges, which contribute to caking, can be identified through XRD analysis of the fertilizer compounds.
It is crucial to keep in mind that when different salts are blended together, their resulting CRH characteristics will differ from those of the individual salts within the blend. Therefore, when developing a blend with the desired NPK formula, it is important to consider the compatibility of the different salts with each other. The combined salts should have a resulting CRH significantly higher than the ambient air humidity to prevent moisture absorption and caking. If fertilizer is placed in an atmosphere with humidity above its critical humidity, it begins to absorb the moisture and the caking mechanism is activated. Furthermore, the salts in the blend should be chemically compatible to ensure that they do not react with each other and cause caking.
When dealing with fertilizers that are prone to caking, it is also important to consider the pressure exerted on the fertilizer particles during extended periods of storage. This is because pressure can increase the contact area between granules, leading to more breakages and potential caking. To reduce the pressure on the fertilizer, it may be necessary to limit the number of bags stacked or the height of bulk piles. By doing so, the risk of caking can be minimized and the quality of the fertilizer can be preserved.
KNOW-HOW
CRITICAL RELATIVE HUMIDITY AND CHEMICAL COMPATIBILITY
The primary factor that impacts the kinetics of caking is the difference between the ambient humidity and the CRH of the fertilizer. To better understand this relationship, tables featuring the individual CRH of various fertilizers, the CRH of fertilizer blends, and the chemical compatibility of different fertilizer salts are provided below. These tables can aid in the development of effective strategies for preventing caking and maintaining the quality of fertilizers during storage.
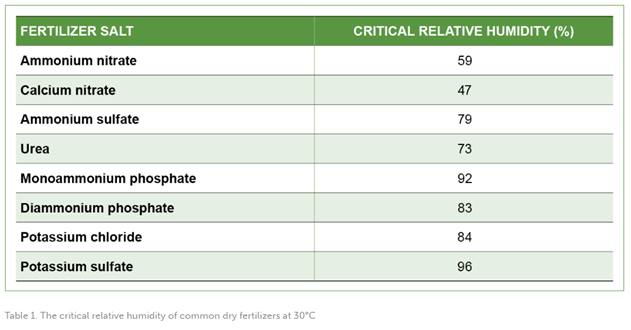
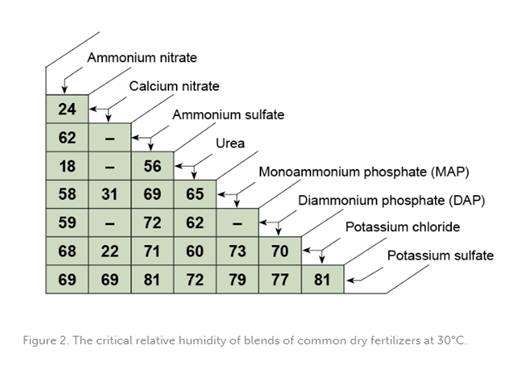
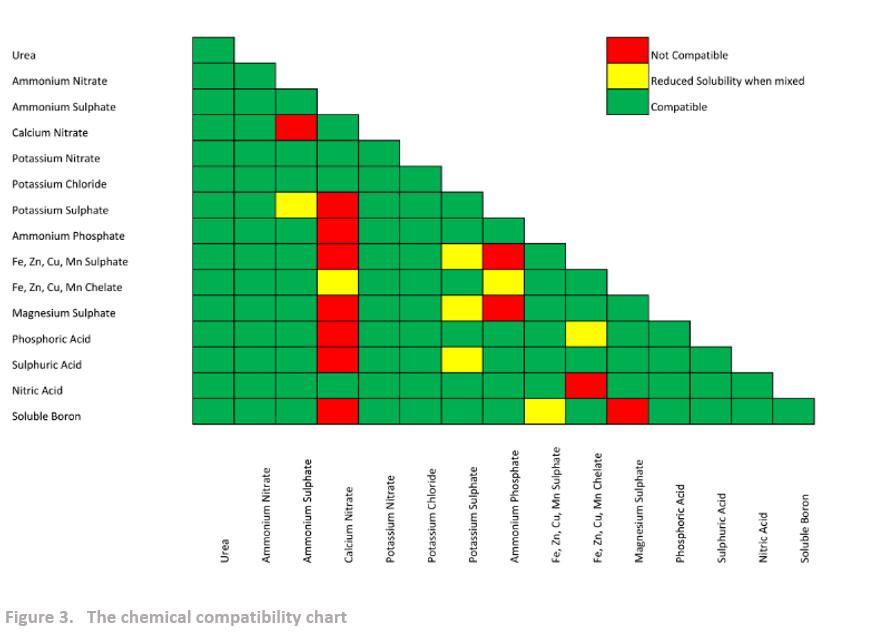
CONCLUSIONS
- In conclusion, a proper CRH of the combination of salts in a fertilizer blend is of critical importance in preventing caking of NPK product and so achieving optimal results in agricultural practices.
- Proper CRH considerations help prevent caking by ensuring that the salts in the blend have similar moisture-absorbing properties. This allows the fertilizer to remain free-flowing and maintain its powder form, facilitating accurate and uniform application. In addition to preventing caking, proper CRH also minimizes the risk of nutrient losses during storage and transportation, as well as in the field.
- The chemical compatibility of salts refers to their ability to remain stable and not react with each other when combined in a fertilizer blend. When salts are incompatible, they can react with each other, resulting in the formation of undesirable compounds or causing nutrient losses, which can adversely affect plant growth and yield.
- Chemical compatibility is essential because it ensures that the desired nutrients are delivered to the plants effectively without any negative interactions. Different salts have varying levels of compatibility with each other, and it is crucial to consider these factors when formulating a fertilizer blend. Compatibility issues can arise due to factors such as pH, cation and anion interactions, solubility, and precipitation reactions.
A well-balanced fertilizer blend with compatible salts and proper CRH ensures that the essential nutrients are provided to plants in the right proportion, promoting healthy growth, development, and optimal yield. It also helps prevent nutrient imbalances and losses that can lead to nutrient deficiencies or toxicities in plants.