Abstract
This article clarifies the commonly misunderstood color instability in aqueous SC formulations caused by the chemical structure of pesticide molecules. The article focuses on addressing such problems associated with very common pesticides. The author proposes to help in resolving quality and production issues with color instability, contact the author.
Aqueous SC (Suspension Concentrate) formulations are widely used for pesticide applications. These formulations consist of pesticide particles suspended in water. It is well known that color instability is a common phenomenon in SC formulations. However, it is often not recognized that this instability can be attributed to chemical reactions occurring between the pesticide molecules and other formulation components or environmental factors.
In particular, the color instability of SC formulations in aqueous medium is often due to the presence of certain functional groups and conjugated double bonds in pesticide molecules. These features make the molecules highly susceptible to oxidation when exposed to air or other oxidizing agents. Additionally, pH-induced reactions, which involve acid - base chemistry, can occur when acidic or basic components in the formulation interact with the pesticide molecules. These interactions can alter the electronic structure and the presence of chromophores of the pesticide molecule, ultimately influencing its color. The color changes can range from a shift in hue to another color or even to a complete loss of color intensity. Understanding the role of chemical reactions in color instability is crucial for addressing this issue. Let's break it down.
Conjugated double bonds: Pesticide molecules with conjugated double bonds have a specific arrangement of alternating single and double bonds. This conjugated system can absorb and interact with light, resulting in distinct colors. With every double bond added, the system absorbs photons of longer wavelength, and the compound ranges from yellow to red in color. The presence of conjugated double bonds makes these molecules susceptible to changes in their electronic structure, which can be influenced by factors such as pH and oxidation.
pH changes: Pesticide molecules with conjugated double bonds can undergo conformational changes in response to pH variations. pH changes can affect the protonation or deprotonation of functional groups, altering the electronic distribution within the molecule. These changes in molecular structure shift the electronic configuration and influence the absorption and reflection of light. As a result, the color of the pesticide molecule or formulation can change when exposed to different pH conditions.
Oxidation: Oxidation refers to the chemical process where a molecule loses electrons. Pesticide molecules with conjugated double bonds can undergo oxidative reactions when exposed to air or other oxidizing agents. Oxidation can disrupt the conjugated system, leading to changes in the electronic structure and resulting in color instability.
Overall, the problem of color instability in aqueous SC formulations of pesticide molecules with conjugated double bonds highlights the importance of considering pH changes and oxidation when formulating and storing these pesticides. It is crucial to understand the chemical properties of these molecules to ensure their stability and efficacy in agricultural or pest control applications.
Now, let's consider a typical color instability problem that arises with commonly used pesticide molecules. Regrettably, the root cause of this problem is frequently misunderstood, resulting in substantial losses due to quality issues. It is essential to comprehend the chemical nature of the problem to effectively address and eliminate it. By accurately identifying and resolving the underlying factors contributing to color instability, businesses can uphold product quality and maintain a strong reputation in the market.
KNOW-HOW
Examples of Pesticide Molecules with Conjugated Double Bonds and
Well-Known Color Instability Issues
ANILINE DERIVATIVES
dinitro aniline:
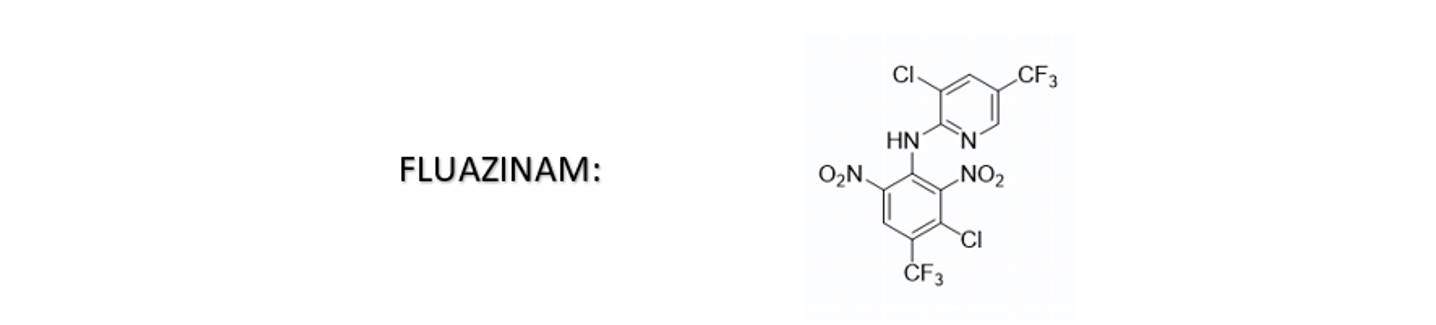
Fluazinam is a pesticide belonging to the chemical class of dinitro anilines, which contains conjugated double bonds. The presence of conjugated double bonds renders fluazinam sensitive to pH changes, resulting in the formation of various conformations that exhibit different colors. In the upper layer of an aqueous SC formulation, the interplay between oxidation and pH changes can induce a color transformation from yellow to red.
anilino pyrimidine:
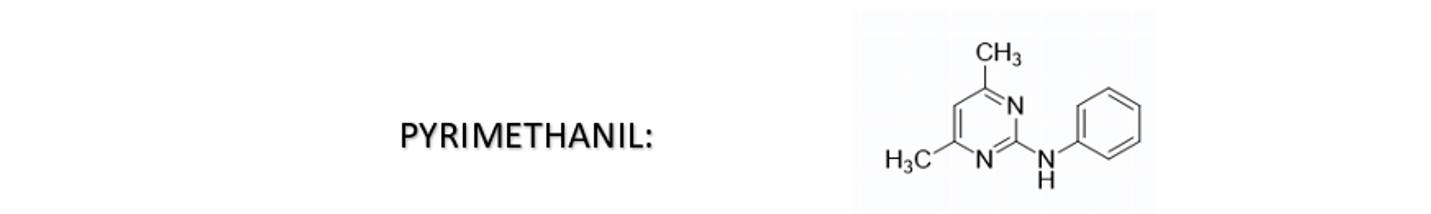
Pyrimethanil is a fungicide belonging to the chemical class of anilino pyrimidines that possesses conjugated double bonds. It is susceptible to pH changes and oxidation, which can cause color changes in aqueous SC formulations. The color may shift from yellow to brown or reddish-brown.
TRIAZOLES
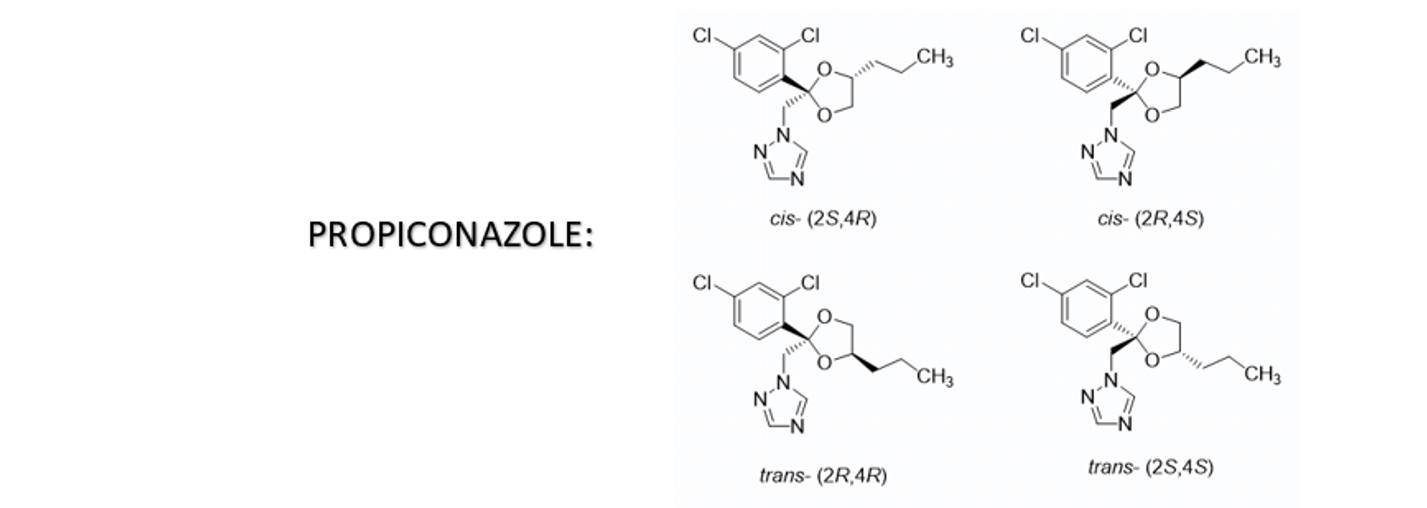
Propiconazole is a triazole fungicide that contains conjugated double bonds. Due to pH changes and oxidation, leading to color instability in aqueous SC formulations, the color may shift from yellow to brown or reddish-brown under different conditions.
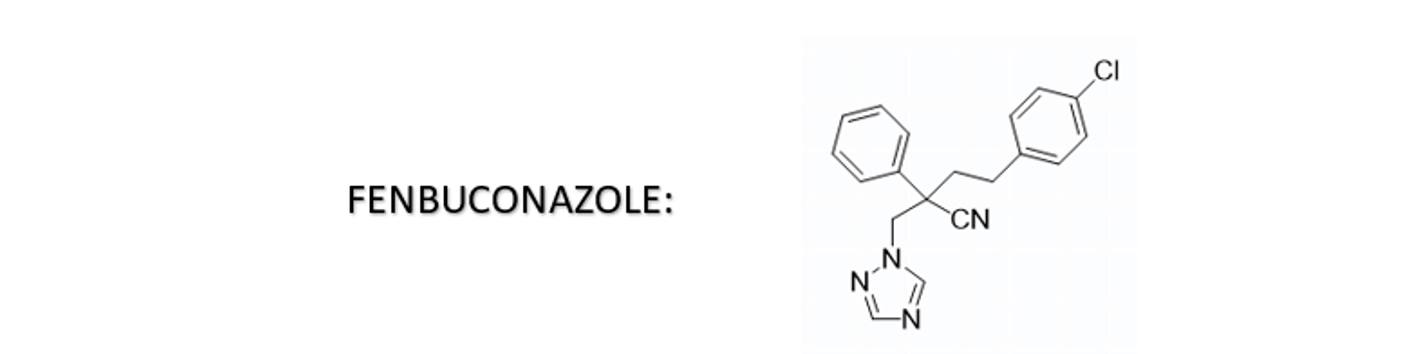
Fenbuconazole is a triazole fungicide that contains conjugated double bonds. It is sensitive to pH changes and oxidation, resulting in color instability in aqueous SC formulations. The color may vary from yellow to brown or reddish-brown.
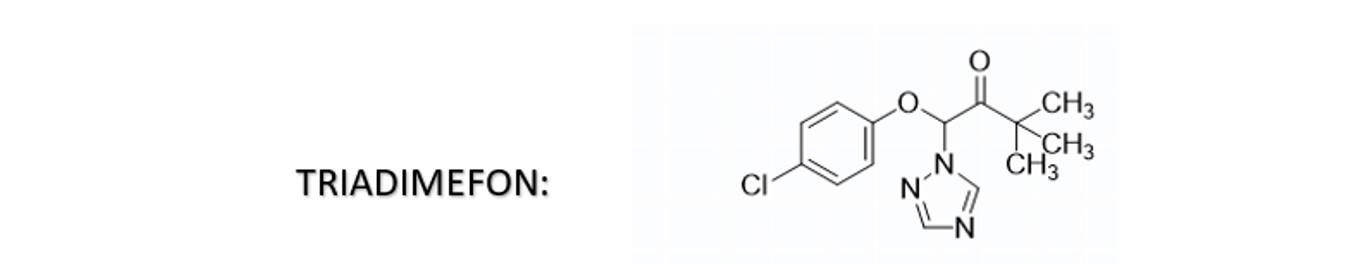
Triadimefon is a systemic fungicide from the class of triazoles. It possesses conjugated double bonds and is known to be sensitive to pH changes and oxidation. In aqueous SC formulations, it can experience color variations, such as from light yellow to brown or reddish-brown, due to these factors.
STROBILURINS

Azoxystrobin is a fungicide belonging to the class of strobilurins. It is Methoxy acrylate that contains conjugated double bonds and is susceptible to pH changes and oxidation. In aqueous SC formulations, it can exhibit color instability, ranging from light yellow to brown or reddish-brown, depending on the conditions.

Trifloxystrobin is a strobilurin fungicide that possesses conjugated double bonds. It is Oxyimino acetate and it’s sensitive to pH changes and oxidation, which can result in color changes in aqueous SC formulations. The color can range from yellow to brown or reddish-brown, depending on the specific conditions.
Here are a few additional examples of widely used pesticide molecules with conjugated double bonds that are sensitive to pH changes and oxidation, leading to color instability in aqueous SC formulations.
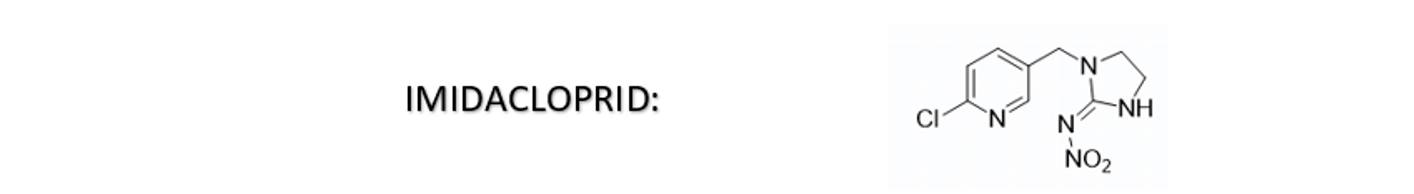
Imidacloprid is a neonicotinoid insecticide. It is Nitroguanidine and it contains a conjugated double bond system. This molecule is known to be sensitive to pH changes and oxidation. In aqueous SC formulations, it can undergo color changes, ranging from yellow to brown or even black, due to these factors.
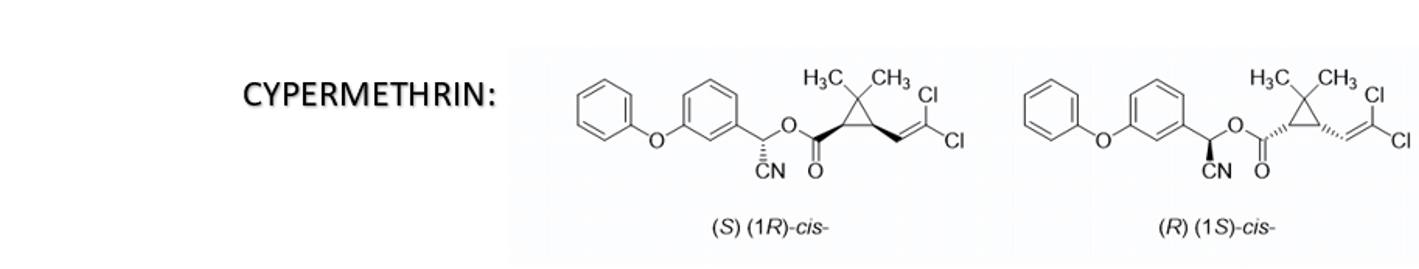
Cypermethrin is a synthetic pyrethroid insecticide that possesses conjugated double bonds. It is sensitive to pH changes and oxidation by air, which can cause color changes in aqueous SC formulations. The color may range from yellow to brown or even dark brown.

Propargite is an acaricide and miticide. It is Sulfite ester that contains conjugated triple bonds. It is sensitive to pH changes and oxidation, which can lead to color variations in aqueous SC formulations. The color may range from yellow to reddish-brown or even dark brown.
These examples demonstrate how pesticide molecules with conjugated double bonds can be susceptible to pH changes and oxidation, leading to color instability in aqueous SC formulations. The specific color changes observed depend on factors such as pH levels, oxidation conditions, and other formulation components.
KNOW-HOW
COLOR STABILIZING STRATEGY
To achieve color stability in SC pesticide formulations containing active ingredients with conjugated double bonds, the following strategies can be considered:
- Formulation Optimization: Carefully select and optimize the formulation components to minimize interactions with the AI molecules. This includes choosing appropriate solvents, surfactants, and stabilizers that are compatible with the AI and minimize chemical reactions that may lead to color changes
- pH Control: Maintain a stable and controlled pH environment within the formulation. Adjust the pH to a level where the AI molecule is least prone to undergo pH-induced reactions that can alter its color. This may involve selecting buffering agents or adjusting the pH through formulation design.
- Antioxidant Incorporation: Include antioxidants in the formulation to inhibit oxidation reactions. Oxidation is a common cause of color instability in AIs with conjugated double bonds. Antioxidants can help prevent or delay the degradation of the AI molecules, thereby preserving their color stability.
- Packaging and Storage: Use appropriate packaging materials that provide protection against light and air. Properly sealed and stored formulations can minimize exposure to environmental factors that may induce color changes in the AIs.
- Stability Testing: Conduct comprehensive stability testing to assess the long-term color stability of the formulation under various conditions, including temperature, light, and storage duration. This will help identify potential issues and guide formulation adjustments to improve color stability.
- Quality Control Measures: Implement stringent quality control measures to ensure consistent formulation production and minimize batch-to-batch variation. This includes strict adherence to manufacturing protocols, regular monitoring of formulation characteristics, and evaluation of color stability during product development and production processes.
- Regular Monitoring and Evaluation: Continuously monitor the color stability of the formulation during its shelf life and adjust if necessary. This includes periodic testing and evaluation of color changes and implementing corrective actions promptly.
By incorporating these strategies, it is possible to enhance color stability in SC pesticide formulations containing AIs with conjugated double bonds, thereby ensuring the quality and reliability of the products throughout their shelf life.